Advancing Science
2021 | 2020 | 2019
2021 Seed Grant Winners
Knowledge Dissemination and Implementation with Patient Partnership Using an Equity, Diversity and Social Justice Lens

NICOLE GEORGE
PhD Student, McGill University
Community Partnerships for Chronic Pain Management: An Equity, Diversity and Social Justice Lens

GHAZAL FAZLI
Post-Doctoral Fellow, Unity Health Toronto
2020 Seed Grant Winners
Advancing the Science of Guideline Development in a Patient-Oriented Research Setting

JILLIAN MACKLIN
MD-PhD Student, University of Toronto

RICHARD HENRY
Post-Doctoral Fellow, McGill University
2019 Seed Grant Winners
Advancing the Science of Patient Engagement in Research

LISA KNISLEY JONES
PhD Student, University of Alberta
Engaging Métis citizens in Manitoba in the development of child health resources
LASHANDA SKERRIT
MD-PhD Student, McGill University
Exploring predictors of women’s overall satisfaction with their HIV care

ALEXANDRA KORALL
Post-Doctoral Fellow, University of Manitoba

AMANDA WURZ
Post-Doctoral Fellow, University of Calgary
Co-creating in-hospital physical activity programming to enhance health for children during treatment for cancer
Honourable Mentions
Huda Shah, University of Toronto (2021)
Carole Lunny, University of British Columbia (2020)
Asset Map of Canadian Clinical Practice Guideline Developers
This interactive asset map provides an inventory of clinical practice guideline developers across Canada.
The Alliance does not have the authority to recommend or endorse any of the guidelines published by the developers. Check with your local health authorities for clinical practices guidelines and recommendations that have been endorsed.
For information on trustworthiness of a guideline, visit the ECRI Guidelines Trust repository.
This database was last updated in April 2018. You can read the full report on how this database was created here.
Share your feedback
We value your feedback, and welcome you to share your experiences in using the Asset Map.
2019 Annual General Meeting – Winnipeg
Our Reports
2023 Annual General Meeting Book
We are pleased to share our fifth Annual General Meeting book highlighting key discussions from the day. The meeting was held virtually on November 16, 2023.
If you have any questions about this meeting book, we welcome you to email us at SPOREA@smh.ca.
2022 Annual General Meeting Book
We are pleased to share our fourth Annual General Meeting book highlighting key discussions from the day. The meeting was held virtually on June 29, 2022.
If you have any questions about this meeting book, we welcome you to email us at SPOREA@smh.ca.
2021 Annual General Meeting Book
The SPOR Evidence Alliance hosted its third Annual General Meeting virtually on June 23, 2021. The meeting convened researchers, trainees, patient/public partners, and other health system decision-makers who serve in one of the six committees of our governance structure. This book provides a summary of the meeting proceedings.
If you have any questions about this meeting book, we welcome you to email us at SPOREA@smh.ca.
2019 Annual General Meeting Book
The SPOR Evidence Alliance hosted its second Annual General Meeting in Winnipeg, Manitoba on May 29, 2019. The meeting convened researchers, trainees, patient partners, and other health system decision-makers who serve in one of the six committees that make up the SPOR Evidence Alliance’s governance structure. This book provides a summary of the meeting proceedings.
If you have any questions about this meeting book, we welcome you to email us at SPOREA@smh.ca.
2018-2019 Annual Report
This report provides an overview of the SPOR Evidence Alliance’s progress during its second funding year, and the first full year of operation.
If you have any questions about this meeting book, we welcome you to email us at SPOREA@smh.ca.
2018 Annual General Meeting Book
This book provides an account of discussions that took place during the SPOR Evidence Alliance inaugural Annual General Meeting held on November 7, 2018 in Toronto, Ontario.
If you have any questions about this meeting book, we welcome you to email us at SPOREA@smh.ca.
2017-2018 Annual Report
This report provides an account of the SPOR Evidence Alliance’s 1-year journey and key developments to date.
If you have any questions about this meeting book, we welcome you to email us at SPOREA@smh.ca.
Clinical Practice Guidelines Database
This is a database of open-access clinical practice guidelines that have been developed in Canada at the local, provincial, or national level, and published between 1996-April 2018.
Each guideline has been grouped under specific areas of science as defined by the CIHR Institutes.
The Alliance did not perform quality assessments of any of the guidelines found in this database. For information on trustworthiness of a guideline, visit the ECRI Guidelines Trust repository.
The Alliance does not have the authority to recommend or endorse use of any particular guideline. Check with your local health authorities for guidelines that they endorse.
You can read the full report on how this database was created here.
Error
You are trying to load a table of an unknown type. Probably you did not activate the addon which is required to use this table type. |
Glossary
Clinical Practice Guidelines: An evidence-based or consensus-based statement prepared to help improve the quality and consistency of care for specific clinical conditions or situations. They incorporate the latest clinical information based on all available scientific evidence and professional opinions into a framework of best practice to promote the best patient outcomes.
Knowledge Synthesis: A research process that systematically summarizes all important studies on a specific research question to help make sense of the diverse evidence on the topic.
Knowledge Translation: The process of summarizing, distributing, sharing, and applying the knowledge uncovered from research. Our goal is to use knowledge translation to improve the health of Canadians and strengthen the health care system through the use of the most effective services and interventions.
Integrated knowledge translation (iKT): A type of knowledge translation where researchers and knowledge users (e.g. policy makers, clinicians) work together on a research project from the beginning (i.e. determining research questions) to the end (sharing and distribution of research findings). This close partnership between knowledge users and researchers produces knowledge that is more relevant and useful to the end users.
Knowledge User: An individual who is likely to use research findings to make informed decisions about health policies, programs and/or practices (e.g. clinical practice guideline producers, policy makers, professional organizations, health system managers).
Patient Engagement: Meaningful and active partnership of patients in all spectrum of research, including governance, priority setting, conduct of the research, and knowledge translation. It may also involve others who bring the collective voice of specific affected communities.
Patient-Oriented Research: Research that includes patients as partners in research and focuses on patient-identified research priorities with the ultimate goal of improving patient outcomes. This type of research is carried out by a diverse team of experts in partnership with patients and other relevant stakeholders, with the goal to speed up distribution, sharing, and application of knowledge generated from research activities.
Patient Partner: An overarching term inclusive of patients (an individual with a health condition), family members of a patient (e.g. parent, spouse, adult child or other close family member of a patient not capable of decision-making), caregivers (family, relative, friends or neighbours providing unpaid assistance to someone with a health condition/limitation), and citizens at large.
Researcher: An individual who is autonomous in their research activities and has an academic or research appointment (e.g. Scientist, Professor).
Research Trainee: A graduate student or post-doctoral fellow who is enhancing their research skills through actual involvement in research and works under the formal supervision of an independent researcher.
Training and Learning
We Build Capacity
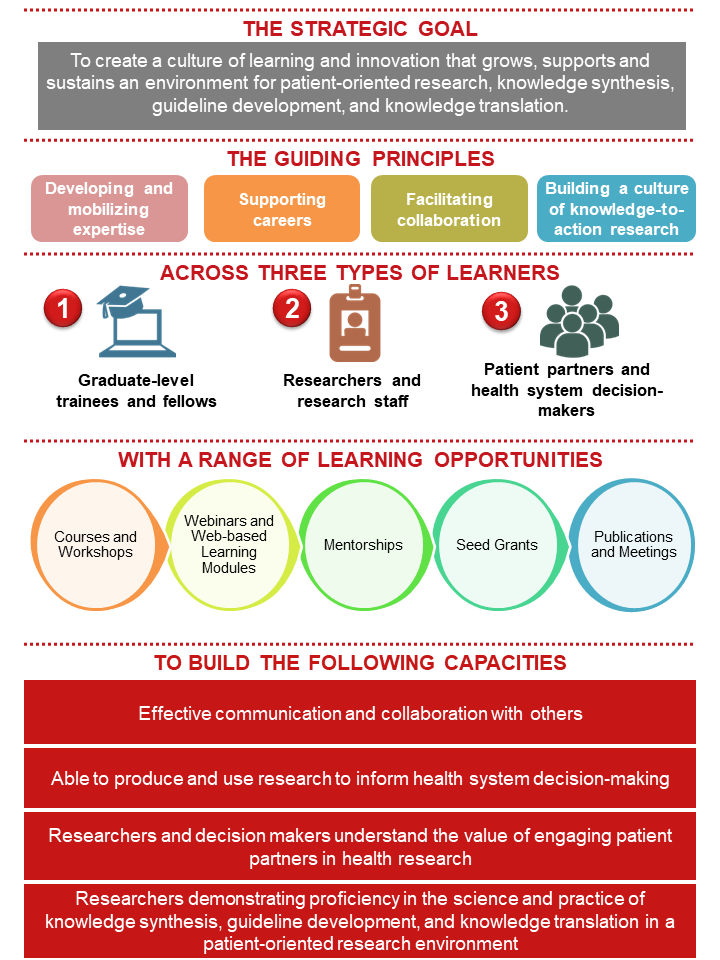
The SPOR Evidence Alliance aims to create a culture of learning and innovation that grows, supports and sustains an environment for patient-oriented research with a focus on knowledge synthesis, guideline development and knowledge translation.
Using the SPOR Capacity Development Framework, our capacity-building strategy was built with consideration for the following guiding principles:
- Developing and mobilizing expertise: Building on the existing diverse set of expertise and skills of the research community to strengthen the capacity of patient-oriented research by providing opportunities for training, collaboration and mentorship in knowledge synthesis, guideline development and knowledge translation.
- Supporting careers: Creating supportive environments that include training and mentorship opportunities.
- Facilitating collaboration: Supporting a collaborative, interdisciplinary research environment and fostering integration, respect and mentorship among researchers, patient partners and health system decision-makers.
- Building a culture of knowledge-to-action research: Creating a culture for demand-driven and context-sensitive research, and enhancing capacity to translate new knowledge into practice.
Training Opportunities
We offer a range of learning opportunities including courses, workshops, webinars, web-based learning, mentorship opportunities, annual seed grants, and scientific meetings.
All training activities have been tailored to adult learning behaviours that promote an environment for self-directed learning and provide practical material relevant to their needs.
These learning opportunities are tailored to three groups of learners:
-
- Graduate-level trainees and fellows, to grow their skills and knowledge in advancing the science and practice of knowledge synthesis, guideline development and knowledge translation in a patient-oriented research environment.
- Researchers and research staff, to build on their diverse expertise and skills in health research and focusing on strengthening their capacity of knowledge synthesis, guideline development, and knowledge translation in a patient-oriented research setting.
- Patient partners and health system decision-makers (e.g., clinicians, healthcare managers and policy-makers), to develop basic knowledge of the science of knowledge synthesis, guideline development, and knowledge translation in a patient-oriented research environment, and how to apply research evidence into practice.
Courses and Workshops
SPOR Evidence Alliance members can participate in the following courses and workshops free-of-charge (limited seats available*):
- Introductory Course on Knowledge Synthesis for Knowledge Users (Course details)
- Foundations of KT (Course details)
- Practicing KT (Course details)
- End of Grant KT (Course details)
- KT Canada Summer Institute (Workshop details)
- See all upcoming courses here
* Seats are intended for trainees (whose supervisors are also SPOR Evidence Alliance members) but non-trainees may be admitted, space permitting.
Our Partners
The SPOR Evidence Alliance is made possible by a five-year grant from the Canadian Institutes of Health Research (CIHR) under Canada’s Strategy for Patient-Oriented Research (SPOR) Initiative, and the generosity of sponsors from 41 public agencies and organizations across Canada who have made cash or in-kind contributions.
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
 |
 |
 |
 |
|
 |
 |
 |
 |
|
 |
 |
 |
||
 |
 |
 |
 |
|
 |
 |
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
The SPOR Evidence Alliance is thankful for the ongoing collaborations from members of 52 Canadian and international public agencies and organizations who have provided letters of support.
SPOR Entities
 |
 |
 |
 |
 |
|
 |
 |
 |
|
 |
|
 |
Government Institutions
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Health Institutions and Organizations
 |
 |
 |
 |
 |
 |
Healthcare Professional Colleges and Societies
 |
 |
 |
 |
 |
 |
 |
 |
Research Alliance and Institutions
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Post-Secondary Institutions
 |
 |
 |
 |
 |
Publishing Institutions
 |
 |
Our Governance
Our Governance Structure
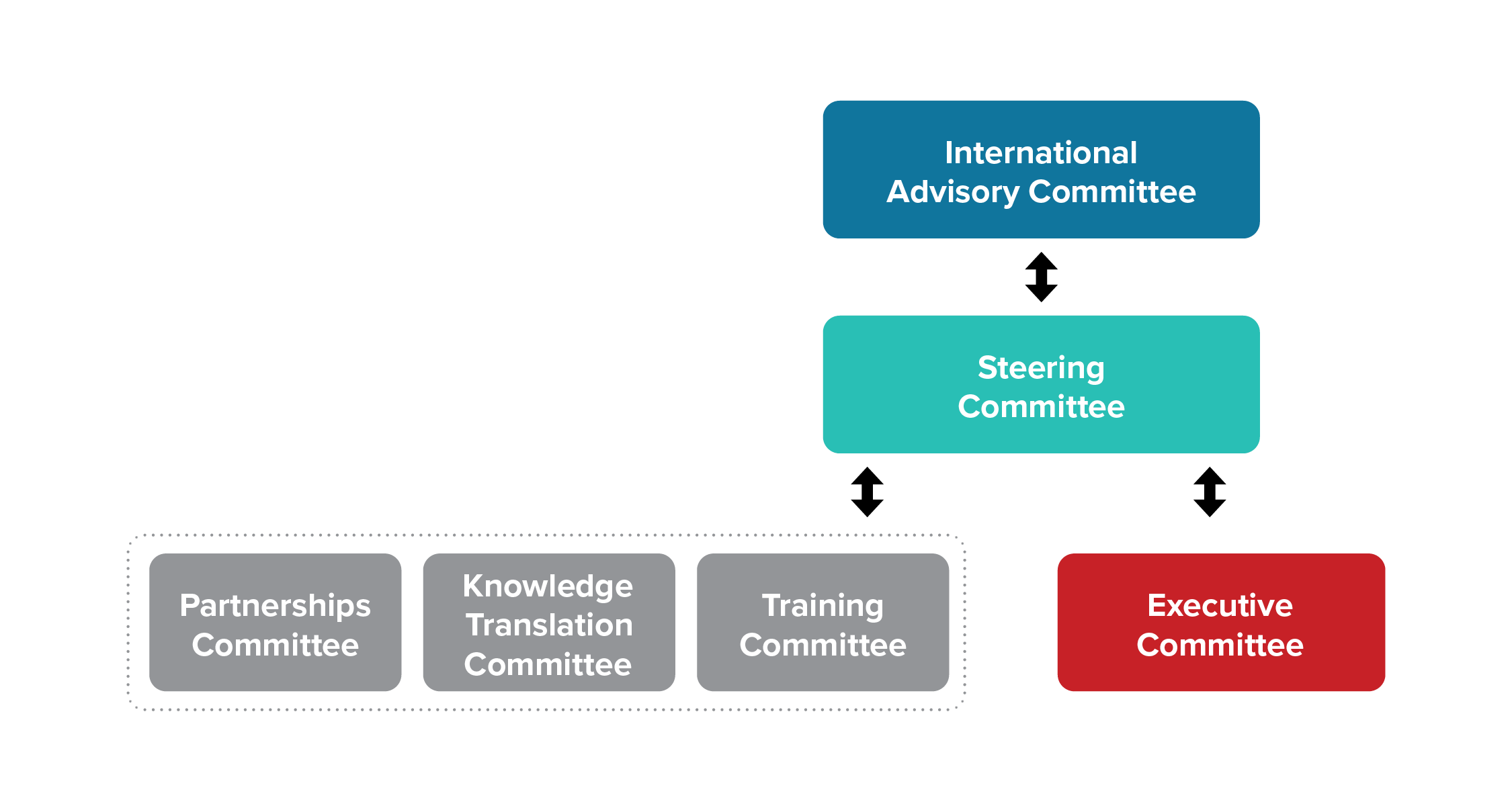
The SPOR Evidence Alliance has six standing committees that play an advisory role to the principal investigators in governing the network. Members within the committees represent other SPOR entities, patient partners, researchers, trainees, government decision-makers, health system managers, healthcare providers, and members of health charities. Each of the six committees operate in a flat structure with specific mandates with specific mandates as per the Terms of Reference. Each committee works in a collaborative environment with cross-communication between committees.
Our Guiding Principles
- We nurture an inclusive governance structure, with an open call to all our members to serve when a seat becomes available.
- We try to ensure diverse representation of members on the basis of several factors, such as gender, geographic location, official language (English and French), and experiences (e.g., researchers, trainees, patient partners, policy-makers, healthcare providers and other decision-makers).
- We provide adequate support and flexibility to ensure patient partners can meaningfully contribute to discussions and decisions. This includes creating welcoming environments that promote honest interactions, cultural competencies, training and education, as well as offering fair financial compensation for time and contribution.
- We promote mutual respect between researchers, practitioners, decision-makers and patient partners, where everyone values each others’ expertise and experiential knowledge.
- We maintain transparency by openly publishing our annual progress and financial reports on our website.
- Our governance structure is fit-for-purpose and has a clearly defined scope and mandates for each standing committee.
How We Manage Conflicts of Interests
- We currently DO NOT accept any funding from private industry (e.g., pharmaceutical companies, medical device manufacturers) to support our research activities.
- All our members must declare annual statements of conflicts and competing interests.
- We encourage and nurture open communication and respectful relationships, and strive to resolve conflicts and competing interests through diplomacy.