Our Expertise
Our Expertise
The SPOR Evidence Alliance specializes in supporting knowledge synthesis, guidelines development, and knowledge translation in a purpose-driven, context-sensitive, and patient-oriented research environment. SPOR Evidence Alliance researchers work collaboratively with decision-makers and other stakeholders to produce health research that is fit-for-purpose, thus avoiding any research waste.
Knowledge Synthesis
A knowledge synthesis (also known as evidence synthesis) uses specific, rigorous and transparent methods to bring together information from multiple studies that have looked at the same topic to make sense of their findings. This allows health policy and practice decisions to be made based on all available scientific information, rather than a single study or expert opinion, which can be misleading.
Our approach is to integrate decision-makers throughout the research process to produce knowledge that is relevant and tailored for decision-maker needs.[note]Gagnon ML. Moving knowledge to action through dissemination and exchange. Journal of clinical epidemiology. 2011;64(1):25-31.[/note]
There are many different approaches to knowledge synthesis. You can use the tool What Review is Right For You? to help you decide which approach would be most useful in answering the research question posed by the decision-maker(s).
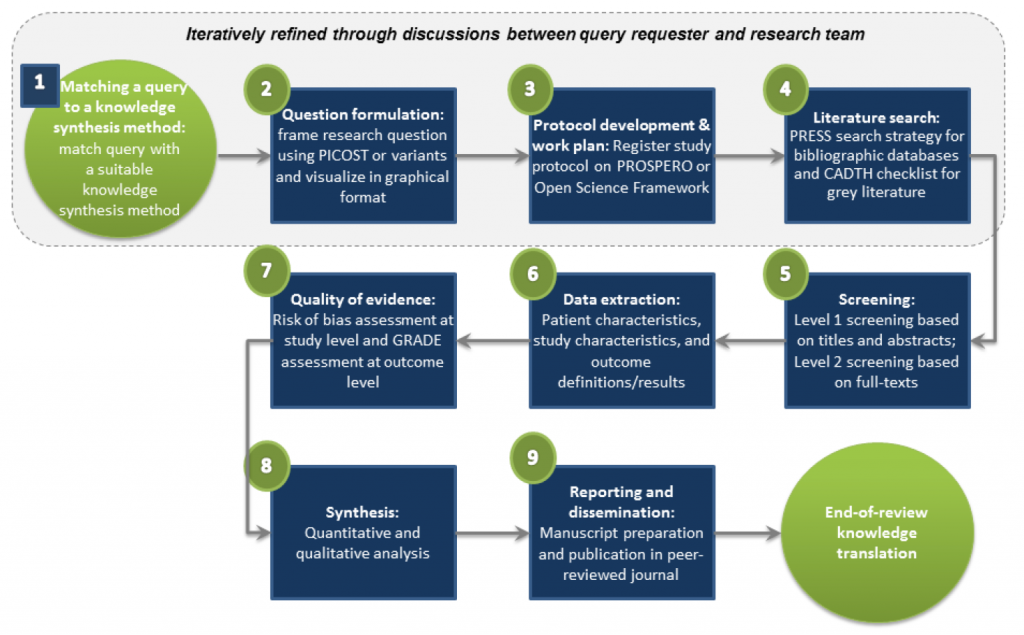
A typical knowledge synthesis approach includes the following 9-step process:
Guidelines Development
A guideline is an evidence-based or consensus-based statement prepared to help improve the quality and consistency of practice for specific clinical or public health conditions or situations. They incorporate the most current information based on all available scientific evidence and professional opinions into a framework of best practices to promote the best patient and population outcomes.
Our goal is to support the development of more patient-centered and end-user-friendly guidelines using an integrated knowledge translation approach.
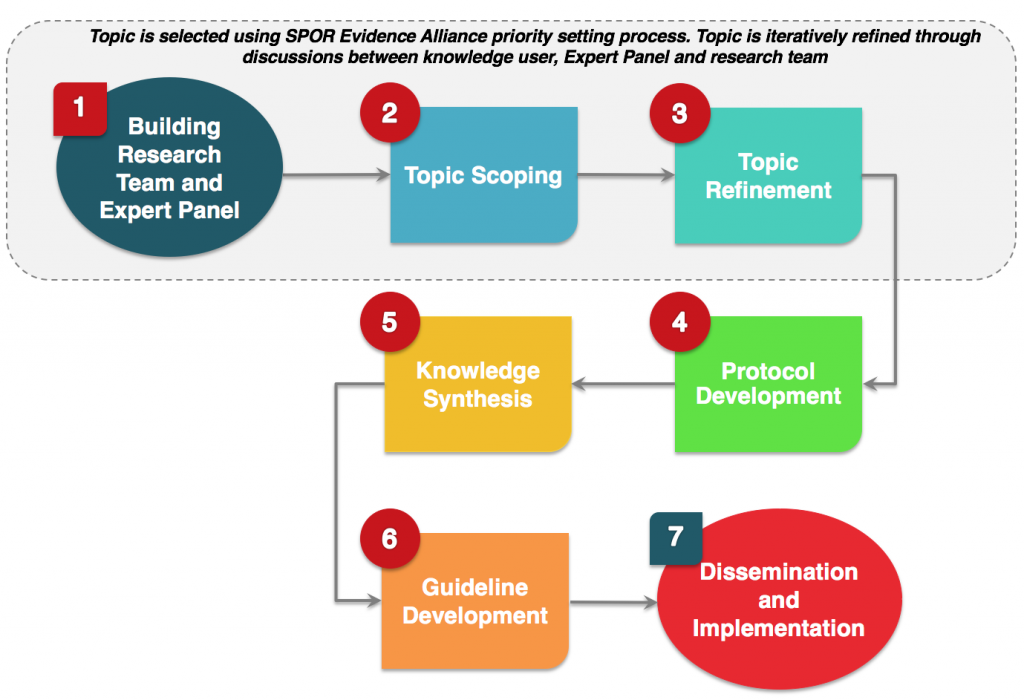
Guidelines are developed using a rigorous methodology and generally include the following 7-step process:
Knowledge Translation
Knowledge translation is the process of summarizing, distributing, sharing, and applying the knowledge uncovered by researchers to improve the health of Canadians, and strengthen the health care system through the use of services and interventions proven to be more effective.
Our philosophy is to use theory and evidence to guide our knowledge translation approach. We publish all knowledge products (such as patient-friendly infographics, plain language summaries, research briefs, and brochures) on our website.
Implementation of guidelines
For all guidelines, an integrated knowledge translation approach is used. This involves engaging all decision-makers and stakeholders to identify the needs and preferences of the end-users by assessing the barriers and facilitators to uptake of the recommendations.
We also use current best evidence to design our strategy for guideline adoption. To identify evidence-informed knowledge translation strategies, we conduct grey literature searches using established databases.